Abstract
Monoclonal Gammopathy of Unknown Significance (MGUS) is characterized as an age-dependent condition that presents with circulating monoclonal protein originating from the clonal proliferation of plasma cells in bone marrow without overt renal failure. However, these proteins have potentials to exert stress on Glomeruli, proximal or distal tubular cells through activation of TOLL-like receptors resulted from excessive endocytosis of light chain proteins, leading to subclinical renal pathologies. This phenomenon lead to forming a new clinicopathological entity named Monoclonal Gammopathy of Renal Significance (MGRS) and can be more pronounced in patients with marginal renal function due to other causes. Importantly, the establishment of MGRS diagnosis is based on renal biopsy which is avoided frequently due to its invasive nature, therefore its overall incidence remains to be defined. Here we aim to define a risk model to predict the rate of worsening renal function in patients with Chronic Kidney Disease (CKD) who have simultaneous MGUS.
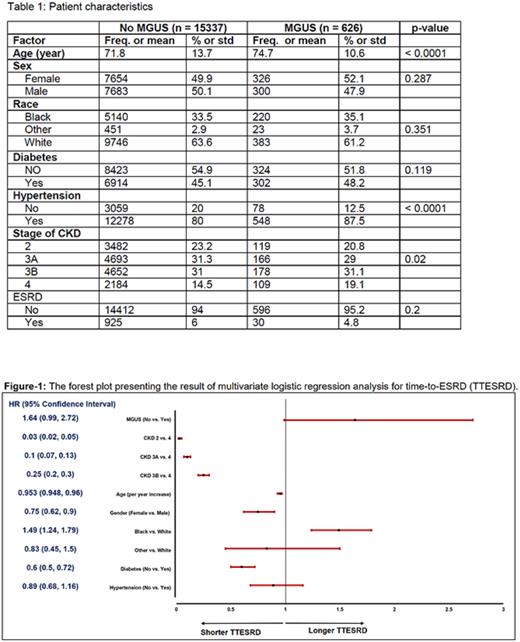
Methods: To define the impact of circulating monoclonal protein on natural course of CKD progression we conducted a retrospective study of CKD patients with MGUS vs. CKD patients without MGUS. Primary end point of the study was Time to End Stage Renal Disease (TTESRD) measured from the date of diagnosis of CKD to the date of diagnosis of ESRD and was censored at the date of last follow-up for those without ESRD with death as competing risk. The Fine and Gray method was used for comparisons of cumulative incidence of ESRD between groups. The effect of important factors on TTESRD was further evaluated using multivariable Fine and Gray method. Survivor distribution was estimated using Kaplan-Meier methods and the difference of OS between/among groups was examined by log-rank test. The difference of continuous variable (age) between group was examined using T-test and the association between two categorical variables was examined using Chi-square test. CKD stages is defined by Glomerular Filtration Rate (GFR) into classic 5 categories as explained before. The rate of stage progression was analyzed as secondary end point.
Results: Patients without valid data (age, date of diagnosis, invalid follow-up data) were excluded for the statistical analysis. The final analysis set included 626 patients with MGUS and CKD and 15337 patients with CKD but without known MGUS. The median follow up time was 19.7 months (range: 0.03, 128). The distribution of CKD stage and other risk factors at both cohorts are illustrated in Table-1. There was significant difference of time-to-ESRD comparing the patients who had CKD alone to patients who had both CKD and MGUS (p = 0.037) in the univariate analysis. After controlling the effects of age, gender, race, CKD stage, diabetes and hypertension, there was still marginally significant difference of TTESRD, comparing the patients who had CKD alone to patients who had both CKD and MGUS (p = 0.056). Specifically, the hazard of having ESRD for patients who had CKD without MGUS was 1.64 times (95% CI: 0.99, 2.72) of the hazard of having ESRD for patients who had CKD and MGUS (see Figure-1).
The CKD stage advancement according to MGUS was analyzes as the secondary end-point of the study. The CKD stage at baseline was compared to the CKD stage at year 1 and year 5 during follow-up. The progression by stage was 28.5% at year 1 for patients without MGUS vs. 29.3% for those with MGUS (p = 0.73). The progression by stage was 38.7% at year 5 for patients without MGUS vs. 39.4 % for those with MGUS (p = 0.89).There was no statistically significant difference between median OS for CKD patients with or without MGUS.
Conclusion: Here we presented the data of around 16,000 CKD pts based on the MGUS diagnosis in 19 years time span and we could not detect any higher trend for CKD pts with MGUS to develop ESRD or move to higher CKD stage. These data can suggest MGRS forms a quite thin slice of the whole MGUS population. Assessing finding of this study in a larger national database is warranted.
Disclosures
Metheny:Incyte: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Gamida: Membership on an entity's Board of Directors or advisory committees. Malek:janssen: Consultancy; BMS: Speakers Bureau; Sanofi: Consultancy; Takeda: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; AdaptiveBio: Consultancy, Honoraria; GSK: Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal